Gleevec - miraculous imatinib
Since its inception in 2001, Gleevec (imatinib) has achieved impressive results in the treatment of chronic leukemia, and is hailed as a major breakthrough in the history of human anti-cancer. Recently, with the release of a movie, the name Gleevec has once again become a hot topic of public discussion.
Cancer and chromosome
Let us first dial the clock back 100 years ago. Scientists at the time knew little about cancer, and mainstream academics simply attributed cancer to viral infections or environmental factors. But a scientist named Theodor Boveri doesn't think so. In animal experiments, some physiologists have discovered abnormalities in mitosis that cause sea urchins to appear similar to cancer attacks. Based on these observations, Theodore also made several assumptions:
- The genetic material of tumor cells may be unstable
- Tumors may be developed from a single cancer cell
- Chromosomal abnormalities cause uncontrolled growth of tumor cells
- The genetic changes that cause the tumor occur on a smaller scale than the chromosome, so we cannot observe it through the microscope.

▲ Theodore's hypothesis is very forward-looking (Source: See page for author [Public domain], via Wikimedia Commons)
With the current understanding of cancer, it is not difficult to find that Theodore's assumptions a hundred years ago are so forward-looking. But his flash of light is too advanced - limited by the limitations of scientific research, until 1921, scientists also believe that there are 48 chromosomes in the human genome. It is conceivable that Theodore's anachronistic genius thoughts are not destined to be verified and recognized by the times.
A few decades later, with the rise of cytogenetics, scientists realized that chromosomes may also be associated with disease: they found that patients with Down syndrome had three chromosomes 21, and Turner syndrome. Patients with partial or complete loss of their X chromosome. Do these cases clearly demonstrate that chromosomal abnormalities can cause disease, and is that cancer also a consequence of chromosomal abnormalities?
At first, the scientists did not reach a consensus. In the case of leukemia, some oncologists only roughly analyzed them and then announced that they could not find any special chromosomal abnormalities. In 1960, a famous scholar at that time asserted after extensive analysis that in most human tumor cells, chromosomes are normal.
However, a paper published in the journal Science in the same year brought an earthquake to the field of cancer research.

▲ Short three paragraphs of words have forever changed human understanding of leukemia (Source: CMLeukemia)
Philadelphia chromosome
In 1956, a young man named Peter Nowell retired from the Navy and returned to his hometown of Philadelphia. There, he joined the Department of Pathology at the University of Pennsylvania, where he focused on leukemia and lymphoma research.

▲ Professor Peter Novell, one of the discoverers of Philadelphia chromosomes (Source: University of Pennsylvania Memorial Website)
Peter also wants to discover the relationship between chromosomes and blood cancer. Unlike many researchers at the time, he decided to label chromosomes with a novel staining technique to see their structure more clearly—the cells first grow on the glass sheet to a certain stage and then bubble up in the water. Subsequently, Giemsa Staining, which infiltrates the cells, allows the chromosomes to emerge from the cells.
"At the time, I didn't know anything about chromosomes," Peter answered in an interview many years later why he wanted to observe chromosomes: "I just thought I shouldn't give them aside."
The course of human history will always be promoted inadvertently. Peter's curiosity quickly brought gains. Not long after, he and his graduate students discovered an interesting phenomenon: in cancer cells of patients with chronic myelogenous leukemia (CML), chromosome 22 is obviously shorter.
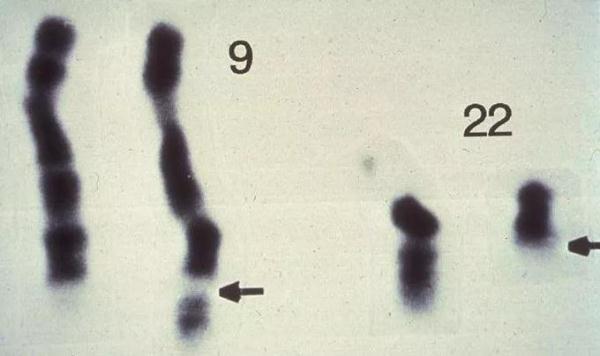
▲The chromosome 22 of some leukemia patients should be significantly shorter (Source: 《Nature》)
Researchers are keenly aware that this chromosomal abnormality may be the underlying cause of such chronic leukemia. In the follow-up study, the researchers further observed the chromosomes of seven leukemia patients, each with a short chromosome 22. In the next few years, Peter and his colleagues published several papers in succession to announce their findings to the world.
"Every typical case, every cell carries this mutation," Peter said. "For me, this means two things. First, this genetic variation is crucial for this type of cancer attack; second, These tumors are indeed grown from individual cells that have mutated."
The whole field of cancer research is shaking! In recognition of this important discovery made by the University of Pennsylvania, the abnormal chromosome 22 in the patient's body was also named "Philadelphia chromosome." On the second day of Christmas 2016, the 88-year-old Peter ushered in the end of his life. Born in the suburbs of Philadelphia, he also died in the suburbs of Philadelphia. Because Peter, the city only a few miles away from him, has always left his own name in the history of human cancer.
Looking for causes of cancer
The discovery of Philadelphia chromosomes is an important breakthrough in the field of leukemia research, but it is not the end. On the contrary, it does not even take the first step on the road of new drug research and development. By the phenomenon of chromosomal abnormalities, we still do not know the pathogenesis behind it. If we don't even figure out the root cause of the disease, how can we treat it?
In 1973, Professor Janet Rowley of the University of Chicago made another step forward based on Peter's discovery. Her team found that the Philadelphia chromosome was short because of the translocation of chromosomes - a partial exchange of human chromosome 9 with chromosome 22, making chromosome 22 short. She is keen to point out that there must be some special carcinogenic mechanism behind this special translocation.
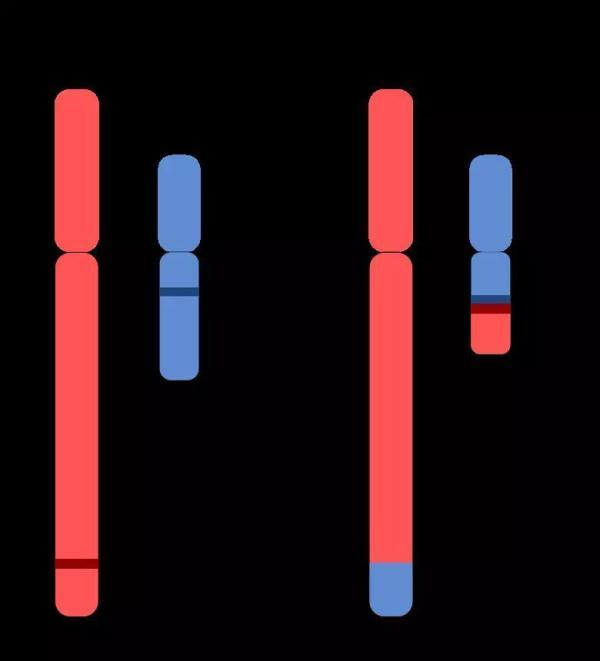
▲ Carcinogenic causes of Philadelphia chromosomes (Source: By Aryn89 [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons)
In order to find this carcinogenic mechanism, we waited for another 10 years.
In 1983, scholars at the National Cancer Institute (NCI) and Erasmus University found that the Abl gene on chromosome 9 coincided with the BCR gene on chromosome 22 due to the interlaced translocation between the two chromosomes. One piece produced a BCR-Abl fusion gene. This fusion gene encodes a peculiar tyrosine kinase. For conventional tyrosine kinases, their activity is tightly controlled and does not suddenly go out of control; however, BCR-Abl proteins are different. It is not controlled by other molecules and is always active. It's like a cell locks up the throttle, causing uncontrolled cell division and causing cancer.
When the researchers introduced the fusion gene into the body of the mouse, the mice developed a fatal leukemia. This finding also finally confirmed that the fusion of BCR and Abl genes is the root cause of such leukemia. This discovery has been a full 30 years since Peter’s paper was published in 1960. Until this moment, human beings have explored enough new knowledge from the unknown field to prepare for the challenge of new drug development.
The long road to new drug research and development
In the late 1980s, scientists at Ciba Geigy (now part of the Novartis Group) launched a series of projects to find protein kinase inhibitors. In a project targeting protein kinase C (PKC), the researchers found that a derivative of 2-phenylamino-pyrimidine exhibits the potential of a drug that inhibits both serine/threonine kinases. With tyrosine kinases. Despite its poor specificity and its inability to be used directly for treatment, it provides a starting point for new drug developers.
Based on this compound, the researchers made a series of synthetic experiments to continuously optimize the properties of this molecule: the addition of a pyridyl group at the 3 position of the pyrimidine can increase its activity in the cell; The added benzamide group can enhance the inhibition of tyrosine kinase; the modification of the 6th position of the anilinobenzene ring further enhances the inhibition of tyrosine kinase; the side chain addition of N-methylpiperazine is extremely The earth has improved the solubility of this molecule, making oral administration possible.
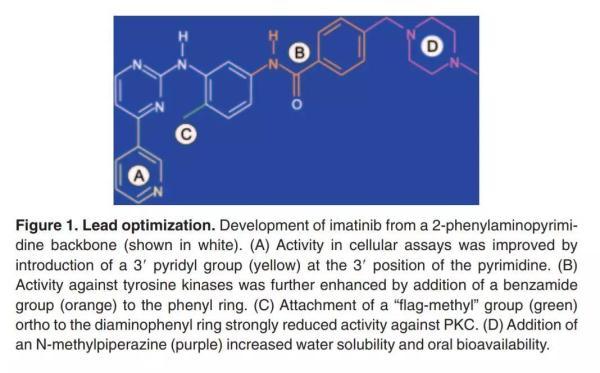
▲ New drug research and development is not a step in the sky, which can not be separated from various designs and optimization (Source: 《Blood》)
After a series of designs and modifications, this molecule demonstrates a high degree of specific inhibition. As long as the cell expresses the BCR-Abl protein, its growth is inhibited by this molecule. Researchers believe it is time to move it to the next stage. The numerator code is CGP57148B, and later has a more resounding name - imatinib.
In the mouse experiment, the researchers further optimized the course and dosage of this molecule. The mice received 11 days of treatment and used 3 times a day. Whether administered intraperitoneally (50 mg per kilogram of body weight) or administered orally (160 mg per kilogram of body weight), cancer was significantly controlled after 48 hours of treatment. On the eighth day after treatment, all treated mice disappeared. Two-thirds (8/12) of the mice did not have recurrence of the disease for the next 200 days. These positive data finally brought Imatinib to the front of the human trial.
Miraculous drug
In June 1998, Imatinib ushered in a historic day – it finally entered the human trial phase.
In this phase 1 clinical trial, the primary goal of the researchers was to find the maximum tolerated dose and explore the safety of the drug. The study enrolled a group of treated patients who were still seriously ill and received daily oral therapy with imatinib. Studies have shown that the drug is not only well tolerated, but also has a miraculous effect: 53 of the 54 patients who received the 300 mg dose had complete hematologic responses (CHR).
This gratifying result quickly brought imatinib to Phase 2 clinical trials. The Phase 2 clinical trial, initiated in 1999, once again validated the positive effects observed in Phase 1 trials. Even more gratifying is that these effects appear to be quite long-lasting: after one and a half years of treatment, the patient's progression-free survival rate still reached 89.2%. Based on its excellent therapeutic effect, the US FDA accelerated the approval of this new drug to treat chronic myelogenous leukemia after Phase 2 clinical trials. The product name of this drug is Gleevec, which we are familiar with.
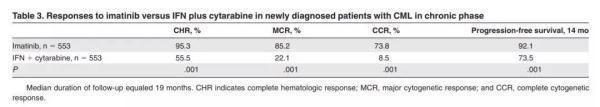
▲ Phase 3 clinical, Gleevec's effect is better than standard therapy (Source: "Blood")
After being approved, the researchers completed the work of Phase 3 clinical trials. Compared to standard therapies, it demonstrates a significant effect on all indicators. Before the birth of Gleevec, only 30% of patients with chronic myelogenous leukemia survived for 5 years after diagnosis. Gleevec increased this figure from 30% to 89%, and after 5 years, 98% of patients still achieved complete hematologic remission. To this end, it has also been included in the World Health Organization's list of essential medicines and is considered to be one of the most effective and safest in the medical system to meet the most important needs.
postscript
In a sense, Gleevec is a true miracle. This is not only because of its incredible efficacy, but also because its success is difficult to replicate. Unlike other cancers, chronic myelogenous leukemia has a single cause. Therefore, it is only a targeted drug that can achieve excellent results. Even so, from the discovery of the Philadelphia chromosome to the accelerated approval of the US FDA, the interval is still 41 years. It is also difficult to develop new drugs.
There is no doubt that Gleevec's research and development story is an excellent example of scientific transformation, and it has also opened a new chapter in targeted cancer treatment. At the moment when anti-cancer innovative therapies are emerging, we should not forget that it is these biologists who have studied basic sciences and the research and development personnel who promoted the emergence of new drugs that have persisted for decades to make life-saving innovative drugs possible. . Here, we also pay a high tribute to them!
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

