FDA approves Akynzeo (fosnetupitant, palonosetron)
Akynzeo is a compound intravenous injection of the 5-HT3 receptor antagonist palonosetron and the NK-1 receptor antagonist fosnetupitant to prevent nausea and vomiting in chemotherapy patients with cancer.
Palonosetron was approved in 2008 for the prevention of nausea and vomiting in the acute phase (within 24 hours) after the start of cancer chemotherapy. Fosnetupitant is a new drug used to prevent nausea and vomiting in the acute and delayed phases (25 hours to 120 hours after chemotherapy) after cancer chemotherapy begins.
On October 10, 2014, the FDA approved a fixed-dose combination of palonosetron and netupitant, an oral capsule formulation developed by Helsinn and also traded under the trade name Akynzeo. This product is also a treatment for the relief of nausea and vomiting in patients with cancer chemotherapy.
Akynzeo's effectiveness is based on two clinical trials involving 1,720 subjects receiving cancer chemotherapy. Subjects were randomized to receive either Akynzeo or oral palonosetron. The two trials were designed to test whether the study drug can prevent vomiting episodes in the acute phase, delayed phase, and overall chemotherapy phase after cancer chemotherapy begins. The first trial showed that 98.5%, 90.4%, and 89.6% of Akynzeo-treated subjects in the acute, delayed, and chemotherapy phases did not develop vomiting or nausea requiring emergency medication. In contrast, subjects treated with oral palonosetron had 89.7%, 80.1%, and 76.5% of the subjects in the acute phase, delayed phase, and overall chemotherapy phase, respectively, without vomiting or nausea requiring emergency medication. The second test showed similar results.
Fosnetupitant is a new molecular entity, Approval date: April 20, 2018
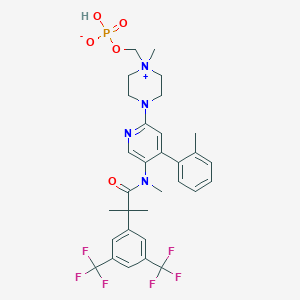
| Name: | Fosnetupitant |
|---|---|
| CAS No.: | 1703748-89-3 |
| Formula: | C31H35F6N4O5P |
| Chemical Names: | Fosnetupitant; UNII-T672P80L2S; 07-PNET; T672P80L2S; 1703748-89-3; Fosnetupitant |
| Molecular Weight: | 688.608 g/mol |
What is the drug for?
AKYNZEO is an injectable drug for the prevention of the nausea and vomiting that happens right away or later in adults receiving certain anticancer medicines (chemotherapy).
AKYNZEO is a combination of two drugs, fosnetupitant and palonosetron. Fosnetupitant is a new drug never before approved by FDA. Palonosetron alone was previously approved by the FDA for the prevention of nausea and vomiting associated with cancer chemotherapy and surgery. Palonosetron is also a component of AKYNZEO capsules that was previously approved by the FDA for the prevention of nausea and vomiting associated with cancer chemotherapy.
How is this drug used?
AKYNZEO is given in combination with dexamethasone (steroid).
AKYNZEO is given by healthcare provider 30 minutes before the start of chemotherapy directly into the vein (IV infusion). It takes about 30 minutes to receive the full dose.
What are the benefits of this drug?
AKYNZEO prevents nausea and vomiting during the first 24 hours and up to 120 hours after chemotherapy.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: AKYNZEO worked similarly in men and women.
- Race: All patients in the trial were White. Differences in how the drug worked among races cannot be determined.
- Age: AKYNZEO worked similarly in patients younger or older than 65 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm607322.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/71544786
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

