FDA approves Apalutamide for non-metastatic castration-resistant prostate cancer
On February 14, 2018, the Food and Drug Administration approved apalutamide (Erleada™, Janssen Biotech, Inc.) for patients with non-metastatic castration-resistant prostate cancer (NM-CRPC).
Approval was based on a multicenter, double-blind, clinical trial (SPARTAN, NCT01946204) randomizing 1,207 patients with NM-CRPC (2:1) to receive either apalutamide, 240 mg orally once daily in combination with ADT (medical castration or surgical castration) (n=806), or placebo once daily with ADT (n=401).
The major efficacy endpoint was metastasis-free survival (MFS). MFS was defined as the time from randomization to the time of first evidence of distant metastasis (new bone or soft tissue lesions or enlarged lymph nodes outside the pelvis), or death due to any cause, whichever occurred first. The estimated median MFS was 40.5 months for patients receiving apalutamide and 16.2 months for those receiving placebo (hazard ratio 0.28; 95% CI: 0.23, 0.35; p<0.0001).
The most common adverse reactions in at least 10% of patients were fatigue, hypertension, rash, diarrhoea, nausea, weight decreased, arthralgia, fall, hot flush, decreased appetite, fracture, and peripheral oedema.
The recommended apalutamide dose is 240 mg (four 60 mg tablets) administered orally once daily.
Full prescribing information is available at ERLEADA™.
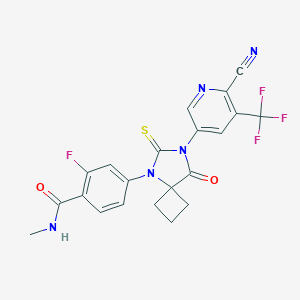
| Name: | Apalutamide |
|---|---|
| CAS No.: | 956104-40-8 |
| Formula: | C21H15F4N5O2S |
| Chemical Names: | ARN-509; 956104-40-8; Apalutamide; ARN 509; JNJ-56021927; ARN509 |
| Molecular Weight: | 477.438 g/mol |
The FDA granted the approval of Erleada to Janssen Pharmaceutical Companies.
URL: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm596796.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/24872560
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

