FDA approves Copiktra ( duvelisib )
The US FDA has approved Verastem's new anticancer drug Duvelisib (Copiktra) for the treatment of adults who have undergone at least two previous treatments, relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma. Duvelisib also received accelerated approval for treatment of adult patients with relapsed or refractory follicular lymphoma.
Duvelisib (IPI-145), a novel oral dual inhibitor of phosphoinositide 3-kinase (PI3K)-δ and PI3K-γ, is used for the clinical treatment of advanced hematological malignancies. Among the 196 subjects who had received at least 2 treatments, the progression-free survival of the Duvelisib group was 16.4 months, and the control group (ofatumumab) was 9.1 months. The overall response rate (ORR) was 78% (Duvelisib) and 39% (ofatumumab), respectively.
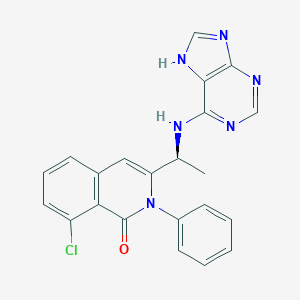
Duvelisib is an orally bioavailable, highly selective and potent small molecule inhibitor of the delta and gamma isoforms of phosphoinositide-3 kinase (PI3K) with potential immunomodulating and antineoplastic activities. Upon administration, duvelisib prevents the activation of the PI3K delta/gamma-mediated signaling pathways which may lead to a reduction in cellular proliferation in PI3K delta/gamma-expressing tumor cells. Unlike other isoforms of PI3K, the delta and gamma isoforms are overexpressed primarily in hematologic malignancies and inflammatory and autoimmune diseases. By selectively targeting these PI3K isoforms, PI3K signaling in normal, non-neoplastic cells is minimally or not affected which would result in a more favorable side effect profile.
| Name: | Copiktra |
|---|---|
| CAS No.: | 1201438-56-3 |
| Formula: | C22H17ClN6O |
| Chemical Names: | IPI-145; INK-1197; (S)-3-(1-((9H-Purin-6-yl)amino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one; UNII-610V23S0JI |
| Molecular Weight: | 416.869 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
COPIKTRA is a drug used to treat adult patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma (FL) who have received at least two prior treatments that did not work or are no longer working.
CLL, SLL, and FL are forms of blood cancer.
How is this drug used?
COPIKTRA is taken as one capsule by mouth twice daily for 28-day treatment cycles.
What are the benefits of this drug?
In adult patients with CLL or SLL, COPIKTRA may delay disease worsening. Patients treated with COPIKTRA lived about 16 months without disease worsening (progression) in comparison to patients treated with comparator drug (ofatumumab) and lived about 9 months without disease worsening.
Among 83 patients with FL who were treated with COPIKTRA, 35 patients (42%) had either complete or partial tumor shrinkage (response).
COPIKTRA for treatment of adult patients with FL was approved under FDA’s accelerated approval program, which provides earlier patient access to a new drug while the company continues to conduct clinical trials to confirm that the drug works well.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: COPIKTRA worked similarly in men and women.
- Race: The majority of patients were White. Therefore, differences in how well the drug works among races could not be determined.
- Age: COPIKTRA worked similarly among patients younger and older than 65 years of age.
URL: https://pubchem.ncbi.nlm.nih.gov/compound/50905713
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm623454.htm
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

