FDA approves fostamatinib tablets for ITP
On April 17, 2018, the Food and Drug Administration approved fostamatinib disodium hexahydrate tablets (TAVALISSE, Rigel Pharmaceuticals, Inc.) for the treatment of thrombocytopenia in adult patients with chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
Approval was based on two identical, double-blind, placebo-controlled trials, FIT-1 (NCT02076399) and FIT-2 (NCT02076412) that enrolled a total of 150 patients with persistent or chronic ITP who had an insufficient response to previous treatment, which included corticosteroids, immunoglobulins, splenectomy, and/or a thrombopoietin receptor agonist. Patients were randomized 2:1 to fostamatinib (100 mg orally twice daily) or placebo for 24 weeks. Dose could be escalated to 150 mg orally twice daily after one month.
Efficacy was based on stable platelet response (at least 50 x109/L on at least 4 of the 6 visits between Weeks 14 to 24). In FIT-1, stable platelet response was demonstrated in 18% (n=9) of patients receiving fostamatinib compared with 0% (n=0) of patients receiving placebo (p=0.03). In FIT-2, stable platelet response was seen in 16% (n=8) and 4% (n=1) of patients, respectively (p=0.26). In the FIT-3 (NCT 02077192) extension study, a stable response was observed in 23% (n=10) of patients newly exposed to fostamatinib. Durable platelet responses were seen in the FIT-1, FIT-2 trials and the FIT-3 extension study.
The most common adverse reactions in at least 5% of patients treated with fostamatinib were diarrhea, hypertension, nausea, dizziness, alanine aminotransferase/aspartate aminotransferase (ALT/AST) increased, respiratory infection, rash, abdominal pain, fatigue, chest pain, and neutropenia. In the ITP double-blind studies, serious adverse drug reactions were febrile neutropenia, diarrhea, pneumonia, and hypertensive crisis, which each occurred in 1% of patients receiving fostamatinib.
The recommended dose initially is 100 mg administered orally twice daily. After a month, if platelet count has not increased to at least 50x109/L, increase dose to 150 mg twice a day.
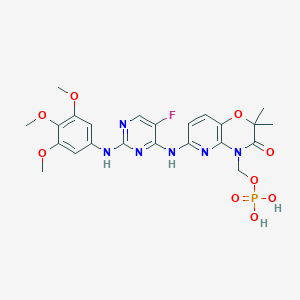
| Name: | Fostamatinib |
|---|---|
| CAS No.: | 901119-35-5 |
| Formula: | C23H26FN6O9P |
| Chemical Names: | Fostamatinib; 901119-35-5; R788; Fostamatinib(R788); Tavalisse; Fostamatinib (R788) |
| Molecular Weight: | 580.466 g/mol |
URL: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm604956.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/11671467
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

