FDA approves Krintafel( tafenoquine)
On July 20, GlaxoSmithKline (GSK) and Medicines for Malaria Venture announced that the US FDA has approved the marketing of its Krintafel (tafenoquine) for the treatment of P. vivaxmalaria.
This is the first new drug to prevent Plasmodium vivax malaria, and the 23rd new drug approved by the US FDA this year.
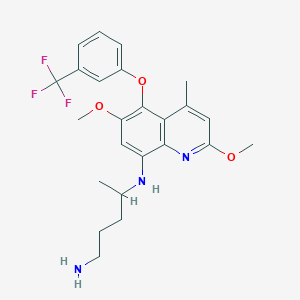
The approved Krintafel (tafenoquine) is a derivative of 8-aminoquinoline that is therapeutically active at all stages of the life cycle of Plasmodium vivax.
In a global comprehensive clinical program, data from 13 studies and 4,000 patients validated their safety and efficacy. Therefore, the US FDA approved the launch of this new drug. Since malaria is a neglected tropical disease, the FDA has also awarded GSK a “Cultural Disease Priority Review Voucher” to motivate its efforts in this area of the disease.
| Name: | Krintafel |
|---|---|
| CAS No.: | 106635-80-7 |
| Formula: | C24H28F3N3O3 |
| Chemical Names: | Tafenoquine; WR-238605; WR 238605; Etaquine; N(4)-(2,6-Dimethoxy-4-methyl-5-((3-trifluoromethyl)phenoxy)-8-quinolinyl)-1,4-pentanediamine |
| Molecular Weight: | 463.501 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
KRINTAFEL is a drug used to prevent relapse of malaria caused by the parasite, Plasmodium vivax. It is used in patients 16 years of age and older, who are already receiving medicine to treat acute Plasmodium vivax malaria infection.
Plasmodium vivax malaria is a serious disease of the blood that is spread by mosquitos infected by a parasite called Plasmodium vivax.
How is this drug used?
KRINTAFEL is administered as a single dose of 2 tablets taken by mouth.
What are the benefits of this drug?
More patients who received KRINTAFEL plus chloroquine (a drug approved for the treatment of acute malaria) were free of disease after 6 months in comparison to the patients who received placebo drug plus chloroquine.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: KRINTAFEL worked similarly in males and females.
- Race: KRINTAFEL worked similarly in all tested races. The number of White patients was limited; therefore, differences in response among the White race and other tested races could not be determined.
- Age: The number of patients older than 65 years was limited. Therefore, differences among age groups could not be determined.
URL: https://pubchem.ncbi.nlm.nih.gov/compound/115358
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm615729.htm
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

