FDA approves Moxidectin
On June 13, the FDA approved moxidectin for the treatment of onchocerciasis (river blindness) in patients 12 years of age and older by oral administration of 8 mg per day (4 tablets of 2 mg tablets).
Onchocerciasis is the second leading cause of blindness in the world following trachoma, mossy dermatitis, subcutaneous nodules and visual impairment caused by parasitic nematodes parasitic on human skin, subcutaneous tissue and eyes. A parasitic disease characterized by river blindness or filariasis.
Moxidectin is a novel anti-parasitic drug, a single-component macrolide antibiotic obtained by fermentation of Streptomyces. Its approval is based primarily on two clinical study data.
In trial 1, 978 patients received Moxidectin tablets at a single oral dose of 8 mg; 494 patients received a single oral dose of ivermectin of approximately 150 mcg/kg. In trial 2, 127 patients received Moxidectin tablets in a single oral dose ranging from 2 mg (this is not an approved dose) to 8 mg (38 recommended 8 mg doses), and 45 patients received a single dose of ivermectin. The oral dose is approximately 150 mcg/kg. The data show that Moxidectin has a significant advantage over current standard treatments (ivermectin) in inhibiting microfilaria in the skin.
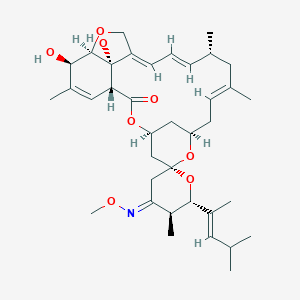
| Name: | Moxidectin |
|---|---|
| CAS No.: | 113507-06-5 |
| Formula: | C37H53NO8 |
| Chemical Names: | UNII-NGU5H31YO9; Cydectin; ProHeart 6; NGU5H31YO9 |
| Molecular Weight: | 639.83 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MOXIDECTIN is a drug used for the treatment of onchocerciasis in patients 12 years of age and older.
Onchocerciasis (river blindness) is an eye and skin disease which is spread by the bite of a fly that is infected with a worm called Onchocerca volvulus.
How is this drug used?
MOXIDECTIN is administered as a single dose of 4 tablets taken by mouth.
What are the benefits of this drug?
Patients treated with MOXIDECTIN had fewer detectable worms in the skin when compared to patients treated with comparator drug ivermectin.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: MOXIDECTIN worked similarly in males and females.
- Race: All patients were Black. Therefore, differences among races could not be determined.
- Age: MOXIDECTIN worked similarly in patients 12 to 18 years of age and those older than 18 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm612705.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/9832912
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

