FDA approves Mulpleta ( lusutrombopag )
Lusutrombopag is an orally bioavailable thrombopoietin receptor (TPOR) agonist developed by Shionogi & Company (Osaka, Japan). TPOR is a regulatory target site for endogenous thrombopoietin, which acts as a primary cytokine to promote megakaryocyte proliferation and differentiation, and affect other hematopoietic lineages as well, including erythroid, granulocytic and lymphoid lineages [A36736]. Thrombocytopenia, which indicates abnormally low levels of platelets, is a common complication related to chronic liver disease. This hematological abnormality, especially in cases of severe thrombocytopenia (platelet count <50,000/μL), creates challenges to patients requiring invasive medical procedures where there is a significant risk for spontaneous bleeding [A36732]. Lusutrombopag binds to the transmembrane domain of TPOR expressed on megakaryocytes, and causes the proliferation and differentiation of megakaryocytic progenitor cells from hematopoietic stem cells [FDA Label]. In September 2015, lusutrombopag received its first global approval in Japan to reduce the need for platelet transfusion in adults with chronic liver disease and thrombocytopenia who are schedule to undergo an invasive medical procedure [A36730]. Lusutrombopag was approved by the FDA on July 31st, 2018 for the same therapeutic indication under the market name Mulpleta. In two randomized, double-blind, placebo-controlled trials, patients with chronic liver disease and severe thrombocytopenia who were undergoing an invasive procedure with a platelet count less than 50 x 10^9/L were administered lusutrombopag orally [L4166]. Higher percentages (65-78%) of the patients receiving lusutrombopag required no platelet transfusion prior to the primary invasive procedure compared to those receiving placebo [L4166]. Lusutrombopag is currently in phase III development in various European countries including Austria, Belgium, Germany, and the UK [A36730].
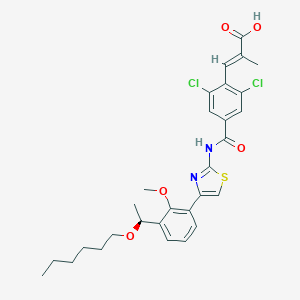
| Name: | Mulpleta |
|---|---|
| CAS No.: | 1110766-97-6 |
| Formula: | C29H32Cl2N2O5S |
| Chemical Names: | LUSUTROMBOPAG; Mulpleta; UNII-6LL5JFU42F; 6LL5JFU42F; S-888711 |
| Molecular Weight: | 591.544 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MULPLETA is a drug used to treat adults with low platelet count who are scheduled to have a medical or dental procedure that could lead to increased bleeding.
MULPLETA is to be used in patients whose low platelet count is the result of long-lasting (chronic) liver disease.
How is this drug used?
MULPLETA is a tablet that is taken 1 time per day for seven days in a row beginning 8-14 days before a scheduled procedure.
What are the benefits of this drug?
Greater proportion of patients treated with MULPLETA did not need a platelet transfusion for bleeding up to 7 days after the procedure, in comparison to patients who received placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: MULPLETA worked similarly in men and women.
- Race: MULPLETA worked similarly in all races.
- Age: MULPLETA worked similarly in patients younger and older than 65 years of age.
URL: https://pubchem.ncbi.nlm.nih.gov/compound/49843517
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm616476.htm
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

