FDA approves Olumiant ( baricitinib )
Rheumatoid arthritis is a chronic, prone to progressive arthritis that is very painful. Currently, TNF inhibitors are a common rheumatoid arthritis therapy, but about two-thirds of patients are unable to get clinical remission from the first treatment. And over time, a large number of patients are unable to maintain the efficacy of the drug. Therefore, these patients urgently need an innovative drug to alleviate the disease.
Olumiant (baricitinib), a new drug jointly developed by Eli Lilly and Incyte, treats adult patients with moderate to severe rheumatoid arthritis who are unable to benefit from TNF inhibitor therapy.
Olumiant is a once-a-day oral JAK inhibitor that efficiently inhibits JAK1, JAK2, and TYK2. In the human body, many cytokines are dependent on the activity of JAK, which has a potential role in the pathogenesis of many inflammatory diseases and autoimmune diseases. By inhibiting the activity of multiple JAKs, Olumiant is expected to bring good news to patients with rheumatoid arthritis.
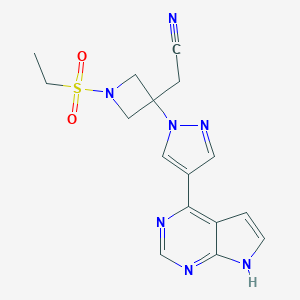
| Name: | Olumiant |
|---|---|
| CAS No.: | 1187594-09-7 |
| Formula: | C16H17N7O2S |
| Chemical Names: | Baricitinib; INCB028050; LY3009104; 2-(3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(ethylsulfonyl)azetidin-3-yl)acetonitrile; INCB 028050 |
| Molecular Weight: | 371.419 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OLUMIANT is a drug for treatment of adult patients with rheumatoid arthritis (RA) whose disease was not well controlled using RA medications called Tumor Necrosis Factor (TNF) antagonists.
How is this drug used?
OLUMIANT is a tablet taken by mouth once daily.
What are the benefits of this drug?
In the clinical trials, a greater proportion of patients who received OLUMIANT achieved an improvement in the signs and symptoms of RA in comparison to patients who received a placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: OLUMIANT worked similarly in men and women.
- Race: OLUMIANT worked similarly among studied races.
- Age: OLUMIANT worked similarly in patients younger and older than 65 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm611018.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/44205240
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

