FDA approves Vizimpro ( dacomitinib )
Lung cancer is one of the most common malignant tumors and the first cause of death in malignant tumors in urban population in China. Non-small cell lung cancer accounts for 80% of lung cancer. EGFR is a protein that helps cells grow and divide. When it is mutated, it can cause cancer cells to form. EGFR mutations occur in 10% to 35% of non-small cell lung cancer worldwide.
In April of this year, the FDA accepted a new drug application for Dacomitinib for first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) carrying EGFR activating mutations. On September 27th, the FDA approved the drug application, which means that a small number of non-small cell lung cancer will be added!
Dacomitinib is a second-generation experimental, oral, once-daily, irreversible, pan-EGFR tyrosine kinase inhibitor (TKI) that is an upgraded version of a first-generation targeted drug. The approval of Dacomitinib is based on positive data from a key phase III clinical study ARCHER-1050.
The study was a global, head-to-head study conducted in 452 patients with locally advanced or metastatic NSCLC carrying EGFR activating mutations and evaluated Dacomitinib (n=227) relative to Gefitinib (n=225) The efficacy and safety of first-line treatment.
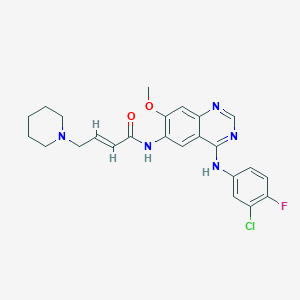
Dacomitinib is a highly selective, orally bioavailable small-molecule inhibitor of the HER family of tyrosine kinases with potential antineoplastic activity. Dacomitinib specifically and irreversibly binds to and inhibits human Her-1, Her-2, and Her-4, resulting in the proliferation inhibition and apoptosis of tumor cells that overexpress these receptors. Dacomitinib, designed as (2E)-N-16-4-(piperidin-1-yl) but-2-enamide, is an oral highly selective quinazalone part of the second-generation tyrosine kinase inhibitors which are characterized by the irreversible binding at the ATP domain of the epidermal growth factor receptor family kinase domains.[A40009] Dacomitinib was developed by Pfizer Inc and approved by the FDA on September 27, 2018.[L4810] Some evidence in the literature suggests the therapeutic potential of dacomitinib in the epithelial ovarian cancer model[A39624], although further investigations are needed.
| Name: | Vizimpro |
|---|---|
| CAS No.: | 1110813-31-4 |
| Formula: | C24H25ClFN5O2 |
| Chemical Names: | Dacomitinib; PF299804; Dacomitinib (PF299804, PF299); PF-00299804; UNII-2XJX250C20 |
| Molecular Weight: | 469.945 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VIZIMPRO is a drug used to treat patients with a type of lung cancer called advanced non-small cell lung cancer (NSCLC). It is to be used as a first treatment in patients whose cancer has spread to other parts of the body (metastatic) and has certain types of gene mutation.
How is this drug used?
VIZIMPRO is a tablet taken by mouth once a day.
What are the benefits of this drug?
Patients who were treated with VIZIMPRO lived about 15 months without disease progression in comparison to patients who receved comparator drug gefitinib who lived about 9 months without disease progression.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: VIZIMPRO worked similarly in men and women.
- Race: The majority of patients were Asians. Therefore, differences in how well the drug work among races could not be determined.
- Age: VIZIMPRO worked similarly in patients below and above 65 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm623149.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/11511120
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

