FDA approves Xerava ( eravacycline )
Eravacycline, known as _Xerava_ by Tetraphase Pharmaceuticals, is a fully synthetic fluorocycline antibiotic of the tetracycline class with activity against clinically significant gram-negative, gram-positive aerobic, and facultative bacteria. This includes most of those bacteria resistant to cephalosporins, fluoroquinolones, β-lactam/β-lactamase inhibitors, multidrug-resistant strains, and carbapenem-resistant Enterobacteriaceae, and the majority of anaerobic pathogens [A38683]. It was first approved by the FDA on August 27, 2018 [L4540]. Eravacycline has demonstrated superior potency to that of antibiotics that are currently being marketed for intraabdominal infections [A38727].
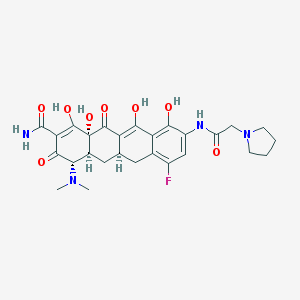
| Name: | Xerava |
|---|---|
| CAS No.: | 1207283-85-9 |
| Formula: | C27H31FN4O8 |
| Chemical Names: | ERAVACYCLINE; TP-434; UNII-07896928ZC; 07896928ZC; Eravacycline [USAN:INN] |
| Molecular Weight: | 558.563 g/mol |
URL: https://pubchem.ncbi.nlm.nih.gov/compound/54726192
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

