FDA approves Zemdri ( plazomicin )
Zemdri (plazomicin) is used in adult patients with complex urinary tract infections (cUTI, including pyelonephritis) caused by certain Enterobacteriaceae bacterial infections with very limited or no treatment options. This drug is an intravenous infusion drug. , once a day.
Zemdri was approved based on a multinational, double-blind, non-inferiority trial in which a total of 609 adult patients with cUTI (including pyelonephritis) were randomly assigned to receive Zemdri (15 mg/kg IV once daily, 30 minutes of infusion) and meropenem (1g intravenously every 8 hours, 30 minutes of infusion) treatment, comparing the comprehensive cure rate of both and the effect of eliminating the proportion of infected microorganisms. The results showed that patients treated with Zemdri had a significant combined cure rate (81.7% vs 70.1%) and a proportion of infected microorganisms (89.5% vs 74.6%) compared with the meropenem group.
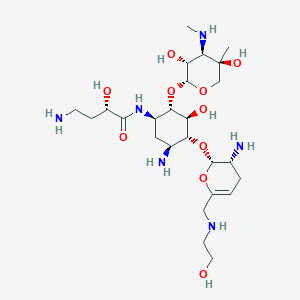
| Name: | Zemdri |
|---|---|
| CAS No.: | 1154757-24-0 |
| Formula: | C25H48N6O10 |
| Chemical Names: | Plazomicin; ACHN-490; UNII-LYO9XZ250J; LYO9XZ250J; D-Streptamine, O-2-amino-2,3,4,6-tetradeoxy-6-[(2-hydroxyethyl)amino]-a-D-glycero-hex-4-enopyranosyl-(1?4)-O-[3-deoxy-4-C-methyl-3-(methylamino)-b-L-arabinopyranosyl-(1?6)]-N1-[(2S)-4-amino-2-hydroxy-1-oxobutyl]-2-deoxy- |
| Molecular Weight: | 592.691 g/mol |
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ZEMDRI is used to treat adults who have a complicated urinary tract infection (abbreviated as cUTI) including infection of the kidneys (pyelonephritis) caused by specific bacteria. It should be used only when few or no other treatment options are available.
How is this drug used?
ZEMDRI is a drug administered by a health care professional directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV, infusion. It is given every 24 hours for 4-7 days.
What are the benefits of this drug?
On Day 5 of IV treatment with ZEMDRI, 88% of patients were cured or had improved signs and symptoms of cUTI and had decreased the number of bacteria in their urine. This was similar to the cure rates for patients who received another antibacterial drug, meropenem (91%).
Because cUTI can come back, many patients continued with oral antibacterial drugs to complete the treatment for cUTI. The benefit was also evaluated after this treatment was completed (about 7-10 days later after total treatment was finished) and it showed that 82% of patients who were initially treated with ZEMDRI were cured from cUTI in comparison to 70% of patients who were initially treated with meropenem.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ZEMDRI worked similarly in men and women.
- Race: Almost all patients were White. The number of patients in other races was limited. Differences in how well the drug worked among races could not be determined.
- Age: ZEMDRI worked similarly in patients below and above 65 years of age.
URL: https://www.fda.gov/Drugs/InformationOnDrugs/ucm612925.htm
URL: https://pubchem.ncbi.nlm.nih.gov/compound/42613186
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

