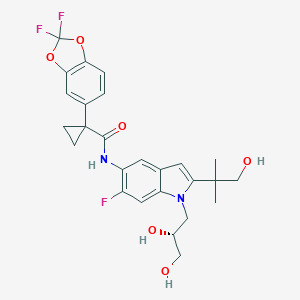
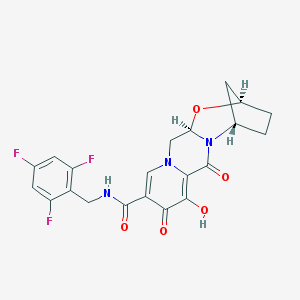
FDA approves Ilumya (tildrakizumab)
What is the drug for?
ILUMYA is a drug for treatment of moderate to severe plaque psoriasis, in adults, who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).