Degradation reactions and stability of Sulfacetamide
Sulfacetamide is stable under normal temperatures and pressures. No dangerous reactions occur below-known conditions of typical use. It is a great important bacteriostatic agent of which is commonly used inside human and veterinary treatments. Therefore it can collect in the environment (mostly surface water).
It has a long lifetime in the surroundings so different degradation reactions are researched:
The photocatalytic degradation of sulfacetamide within water solutions during the lighting of UV radiation together with TiO2 was examined. It absolutely was found that sulfacetamide is usually resistant to biodegradation in addition to that it truly is toxic to the green alga Chlorella vulgaris. It undergoes photocatalytic degradation and the degree of toxicity of the intermediate goods is significantly lower as compared to the initial toxicity. Typically the intermediates can be mineralized in contrast to sulfacetamide.
Sulfonamide → organic more advanced products (degradation) (in presence of OH−).
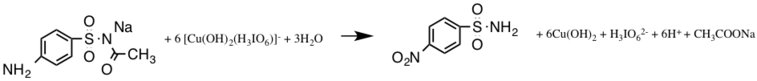
Oxidation regarding sulfacetamide by diperiodatocuperate(lll)
From higher temperatures, sulfacetamide options degrade to its hydrolyzed product, sulphanilamide with the first-order rate constant.
Also, oxidation of sulfacetamide simply by diperiodatocuperate(lll) in an aqueous alkaline medium can take place. Copper(lll) is used since it is involved in many biological electron transfer reactions.
The sulphanilamide may oxidize to an azure product having the first-order effect and it can form the azo dye with a second order reaction.
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

