What is Capsinoids, Where to buy
The Capsinoids compound is a compound derived from the genus Capsicum, and its structural feature is that an unsaturated fatty acid is linked to an alcoholic hydroxyl group of vanillyl alcohol through an ester bond.
In 1989, Yazawa et al. found that a non-spicy pepper variety named CH-19Sweet contains only a small amount of capsaicinoids, but contains a large amount of capsaicinoid-like substances, Kobata according to The difference in Rf values temporarily named the two major spots on TLC as CLS-A and CLS-B. Yazawa et al. hypothesized that capsaicin analogs may be precursor compounds in the biosynthesis of capsaicinoids.
In 1998, Kobata group isolated vanillyl alcohol from CLS-A to separate two compounds from CLS-B and confirmed the structure. The two compounds were named capsaicin Capsiate and Dihydrocapsiate.
In 1999, Kobata group isolated another capsaicin ester compound from CH-19Sweet, named as Nordihydrocapsiate. The naturally occurring capsaicin ester compound in nature is mainly capsaicin ester, although pepper The ester is derived from the genus Pepper, it has no spicy taste, so its pharmacological activity has received extensive attention.

Capsinoids, which includes capsiate, dihydrocapsiate, and nordihydrocapsiate, are substances naturally present in chili peppers. Although they are structurally similar to capsaicin, the substance that causes pungency in hot peppers, they largely lack that characteristic. Capsinoids have an estimated “hot taste threshold” which is about 1/1000 that of capsaicin. Capsinoids were not reported in the scientific literature until 1989 when biologists first isolated them in a unique variety of chili peppers, CH-19 Sweet, which does not contain capsaicin. Capsinoids include capsiate, dihydrocapsiate, and nordihydrocapsiate. Many health effects have been ascribed to capsaicin and capsinoids, both anecdotally and through scientific study, including anticancer, anti-inflammatory, and analgesic activities, and weight management.
Structure
Structural differences between capsaicin and members of the Capsinoids family of compounds are illustrated below. Capsinoids have an ester bond in their structures, as compared with the amide bond characteristic of capsaicin.
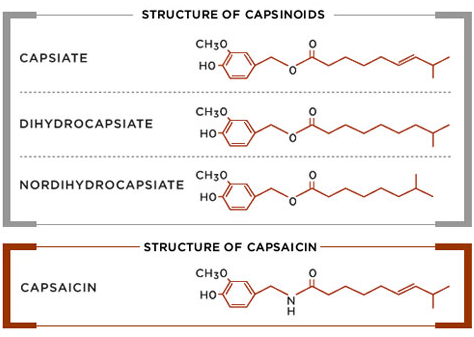
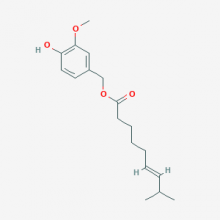
| Name | Capsiate |
| Alias | (4-Hydroxy-3-methoxyphenyl)methyl (6E)-8-methylnon-6-enoate; UNII-PX8I7HG5I3; PX8I7HG5I3; (4-hydroxy-3-methoxyphenyl)methyl (6E)-8-methylnon-6-enoate; Capsiate Natura |
| CAS No. | 205687-01-0 |
| Formula | C18H26O4 |
| Weight | 306.402 g/mol |
| Usage | Pharmaceuticals |
| Appearance | Colorless to light yellow oily liquid or Powder |
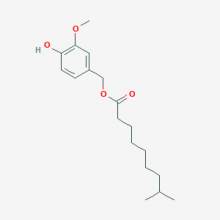

| Name | Dihydrocapsiate |
| Alias | 4-Hydroxy-3-methoxybenzyl 8-methylnonanoate; UNII-W2F7769AEU; Vanillyl 8-methylnonanoate; W2F7769AEU |
| CAS No. | 205687-03-2 |
| Formula | C18H28O4 |
| Weight | 308.418 g/mol |
| Usage | Cosmetics / Pharmaceuticals / Intermediates |
| Appearance | Colorless to the light yellow oily liquid |
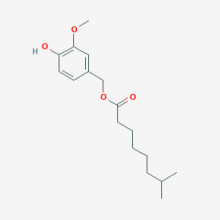
| Name | Nordihydrocapsiate |
| Alias | UNII-4S73H45T4T; 4-Hydroxy-3-methoxybenzyl 7-methyloctanoate; 4S73H45T4T; (4-HYDROXY-3-METHOXYPHENYL)METHYL 7-METHYLOCTANOATE |
| CAS No. | 220012-53-3 |
| Formula | C17H26O4 |
| Weight | 294.391 g/mol |
| Usage | Pharmaceuticals |
| Appearance | Colorless to light yellow oily liquid or Powder |
Mechanisms of action: Capsaicin vs. Capsinoids
It is anecdotally said that hot peppers help people in the tropics “cool off.” This theory is consistent with the peripheral vasodilatory effect of capsaicin that has been shown to lower skin temperature in humans exposed to a hot environment. Capsaicin feels hot in the mouth because it activates sensory receptors on the tongue otherwise used to detect thermal heat. This receptor is called Transient Receptor Potential Vanilloid 1 (TRPV1). TRPV1 receptors are also located in the gut and in other organs. Stimulation of TRPV1 receptors is known to bring about activation of the sympathetic nervous system (SNS). Capsaicin has been shown to increase fat burning in humans and animals through stimulation of the SNS.
Like capsaicin, capsinoids activate TRPV1 receptors, although they are not hot in the mouth. Capsinoids cannot reach the TRPV1 oral cavity receptors, located slightly below the surface in the mouth, because of structural differences from capsaicin. On the other hand, both capsaicin and capsinoids activate TRPV1 receptors in the same manner. Research has indicated that the TRPV1 receptors in the gut are important for the metabolic effects of capsaicin and capsinoids.
Both energy metabolism and body temperature increases are observed in humans following extracted capsinoids or CH-19 Sweet administration. Animal studies also demonstrate these increases, as well as suppressed in body fat accumulation following capsinoids intake. The exact mechanisms and the relative importance of each remain under investigation, as are the effects of capsinoids on appetite and satiation
Major Capsinoids in nature
Capsiate (4-hydroxy-3-methoxybenzyl (E)-8-methyl-6-nonenoate) (CAS No. 205687-01-0)
Dihydrocapsiate (4-hydroxy-3-methoxybenzyl 8-methylnonanoate) (CAS No. 205687-03-2)
Nordihydrocapsiate (4-hydroxy-3-methoxybenzyl 7-methyloctanoate) (CAS No. 220012-53-3)
Data source and Safety testing:
https://en.wikipedia.org/wiki/Capsinoids
https://pubchem.ncbi.nlm.nih.gov/compound/9873754
Where to buy Capsinoids
Our company sells Capsinoids and related ingredients. If you want to know the details of the product, you can reach the relevant page through the above link, or send us an inquiry to tell us the ingredients you want to purchase.
Send inquiry online For more product information and prices
(Pharmaceutical Ingredients Manufacturer & Supplier & Exporter.)
After sending the online inquiry, we will reply you as soon as possible, if not get any response on time please contact us by Tel or Email. —— Green Stone Swiss
Email: sales@raw-pharmaceutical-materials.comTel: +86 592 5365887
WhatsApp: +86 189 6515 7632
Send inquiry online:

